What Problems Bother Patients with Parkinson’s Disease
There have been few comprehensive surveys of the features of the illness bothering Parkinson’s disease (PD) patients, and none have assessed PD patient reports in their own words.
The Making Patients Heard Research Foundation (MPH) has assembled a profile of what bothers PD patients using an extraordinary collection that includes more than 25,000 individuals who reported they had been diagnosed with PD. The data were obtained from the Fox Insight online research study https://foxinsight.michaeljfox.org and its associated shared database FoxDen https://www.michaeljfox.org/fox-den – both sponsored by the Michael J Fox Foundation for Parkinson’s Research. The data (source PD-PROP Feb 2020) were analyzed in collaboration with scientists at Grey Matter Technologies/Modality.ai, and an expert research team including PD clinical specialists, persons living with PD, data scientists, and statisticians.
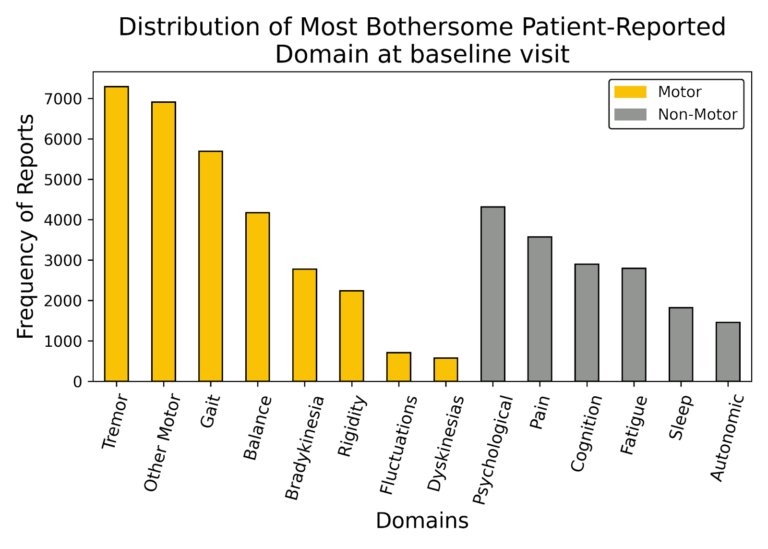
Research participants included 23,164 English-speaking adults (55% men, 45% women) who were asked to report in their own words the problems that bother them due to their PD and how these problems affect their daily functioning. They could report up to five problems and order them starting with the most bothersome. Their online reporting by keyboard entry at study enrollment produced 51,198 verbatim narratives for the most bothersome problem. The verbatim reports were analyzed using natural language processing and human-in-the-loop curation to classify the verbatim text into 14 clinically meaningful domains or topics.

Eight domains were related to the movement disorder of PD (62% reports), and six domains were non-motor (38% reports) representing problems classified as psychological (mood and emotional disorders), pain, cognition, fatigue, sleep, and the autonomic nervous system controlling blood pressure, and gastrointestinal and urinary functions. Most problems in the motor domain were related to tremor, reduced dexterity, handwriting size, and impaired gait and balance.
Further analyses of this dataset will address the 65 individual symptoms that comprise the motor and non-motor domains, as well as the relationships of these symptoms to key factors such as age and duration of PD. The learnings from these analyses will be shared as MPH prepares to launch an innovative online research study designed to follow over time what bothers PD patients, and the impact of these problems on their daily functioning. Additional research technologies will be employed to guide participants and record movement, voice, and other markers of PD. Please stay tuned to this MPH space.